Regulatory Center
Making Telecommunication Tech Permanent in Hospice

The National Association of Home Care and Hospice (NAHC) sent a letter to the administrator of Medicare (CMS) in mid-May urging CMS to “establish
permanent flexibilities allowing hospices to utilize telecommunications to
deliver multi-disciplinary, patient-centered care, and to ensure that such
services can be recorded and monitored for their impact on quality of care.”
Because no one knows when the risks related to COVID-19 will abate, CMS needs to begin discussions about establishing permanent polices regarding the use of telecommunications technology in hospice. NAHC’s recommendations follow:
NAHC’s recommendations follow:
Clarify that hospice providers are permitted to use telehealth
technology in compliance with HIPAA requirements to perform service
visits and as outlined in the patient’s plan of care beyond the term of
the current public health emergency.- Fast-track development of modifiers or revenue codes that can be used
to reflect telehealth encounters for claims reporting. - Clarify that hospice providers can include telehealth encounters on the
Hospice Item Set (HIS) Discharge Section O. - Develop permanent regulations that allow hospices to use telehealth
for face-to-face encounters.
The letter can be found at:
For Your Consideration
FDA Requesting Voluntary Recall of Metformin ER
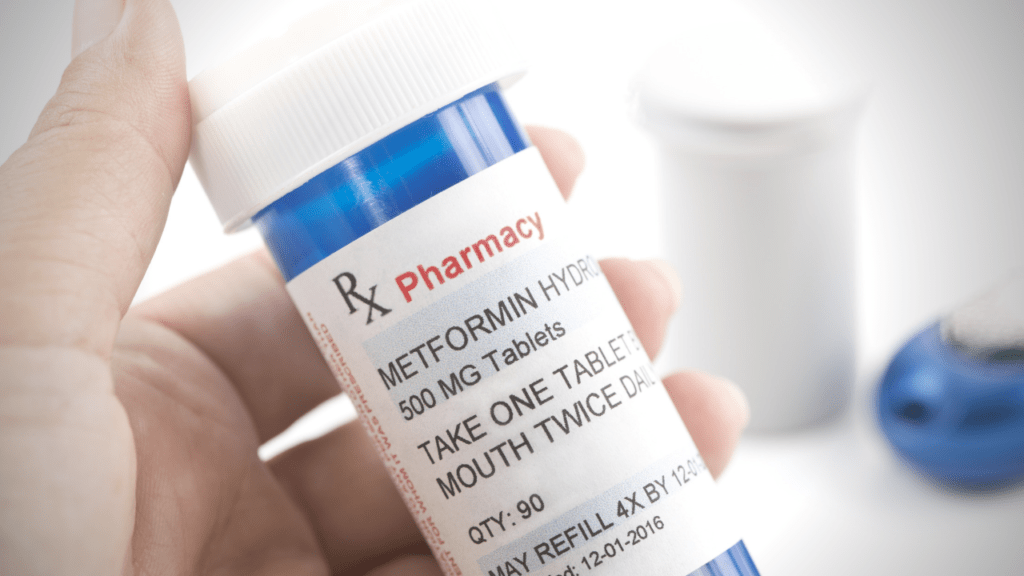
The U.S. Food and Drug Administration is announcing today that agency laboratory testing has revealed levels of the nitrosamine impurity N-Nitrosodimethylamine (NDMA) above the agency’s acceptable intake limit in several lots of the extended-release (ER) formulation of metformin, a prescription drug used to control high blood sugar in patients with type 2 diabetes. The agency is in contact with five firms to recommend they voluntarily recall their products. Company recall notices will be posted on FDA’s website. There are additional manufacturers of the metformin ER formulation that supply a significant portion of the U.S. market, and their products are not being recalled. The FDA is continuing to work closely with manufacturers to ensure appropriate testing. Assessments are underway to determine whether metformin ER recalls will result in shortages and the agency will work closely with manufacturers to prevent or reduce any impact of shortages.
Patients should continue taking metformin tablets even after recalls occur, until they consult with their health care professional who can prescribe a replacement. Patients with type 2 diabetes could face dangerous health risks if they stop taking their prescribed metformin. The FDA recommends that health care professionals continue to prescribe metformin when clinically appropriate; FDA testing has not shown NDMA in immediate release (IR) metformin products (the most commonly prescribed type of metformin). The agency is working with manufacturers of the recalled tablets to identify the source of the NDMA impurity. At this time, the elevated levels of NDMA have been found in some finished-dose tablets of the ER formulation but have not been detected NDMA in samples of the metformin active pharmaceutical ingredient. FDA News Release May 28, 2020
Wise Hospice Options reviewed all patients we served in April and May of this year and found no prescriptions for Metformin ER filled.
In case you were wondering
CDC/CMS Antimicrobial Stewardship Program
Antibiotics are among the most frequently prescribed medications in the frail elderly, especially those residing in long-term care facilities (LTCs). Improving the use of antibiotics in healthcare to protect patients and reduce the threat of antibiotic resistance is a national priority. Antibiotic stewardship refers to a set of commitments and actions designed to “optimize the treatment of infections while reducing the adverse events associated with antibiotic use.” The Centers for Disease Control and Prevention (CDC) recommends that all acute care hospitals implement an antibiotic stewardship program (ASP) the CDC also recommends that all nursing homes take steps to improve antibiotic prescribing practices and reduce inappropriate use.
Although the CDC and CMS recommend the Antimicrobial Stewardship Program (ASP) specifically for hospitals and long-term care nursing facilities the core elements of the ASP may be applicable to many of our hospice patients.
The 7 Core Elements
- Leadership Commitment – Demonstrate support and commitment to safe and appropriate antibiotic use.
- Accountability – Identify physician, nursing and pharmacy leads responsible for promoting and overseeing antibiotic stewardship activities for your organization.
- Drug Expertise – Establish access to consultant pharmacists or other individuals with expertise/training in antibiotic stewardship in your organization.
- Action – Implement at least one policy or practice to improve antibiotic use.
- Tracking – Monitor at least one process of antibiotic use and at least one outcome from antibiotic use among your patients.
- Reporting – Provide regular feedback on antibiotic use and resistance to prescribing physicians, nurses, and other relevant staff.
- Education – Provide resources to physicians, nurses and families/care givers about antibiotic resistance and opportunities for improving antibiotic use.
The full article and detail regarding the core elements of the ASP can be found at the following link:
https://www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html#anchor_1557415462
Updated
Drug Shortage List

These shortages are due either from manufacturing delays or shortage of raw ingredients. This is not an inclusive list, but the listed drugs may be used for hospice patients, especially in the inpatient setting.
Although not in short supply per se, hospitals serving COVID-19 patients are getting priority for the following drugs:
- Chloroquine
- Hydroxychloroquine
- Droperidol
- Dobutamine
For a complete list of drugs on shortage follow this link:
https://www.drugs.com/drug-shortages/
The FDA is warning that there may be an increase in drug shortages due to the effect the coronavirus is having on raw products coming out of China and India used in the manufacturing of drugs.
NOTE: Any items marked with a pill icon are new from the previous month’s newsletter

To close click the X in the top right hand corner.
Drug Shortages
Updated: April 2022
New additions:
Tramadol
| Medication | Type | Date |
|---|---|---|
| Atropine | Inj | 3/10/2022 |
| Bumetanide | Inj | 3/28/2022 |
| Dexamethasone | Inj | 3/11/2022 |
| Diazepam (liq) | Oral | 1/31/2022 |
| Digoxin | Inj | 1/17/2022 |
| Famotidine | Inj/Tabs | 1/18/2022 |
| Fentanyl | Inj | 3/21/2022 |
| Furosemide | Inj | 3/15/2022 |
| Haloperidol | Inj | 3/21/2022 |
| Heparin | Inj | 2/15/2022 |
| Hydralazine | Inj | 3/23/2022 |
| Hydromorphone | Inj | 3/24/2022 |
| Ketamine | Inj | 3/23/2022 |
| Ketorolac | Inj | 3/15/2022 |
| Levetiracetam | Inj/Tabs | 3/22/2022 |
| Lisinopril | Tablets | 3/23/2022 |
| Lorazepam | Inj/Oral | 3/21/2022 |
| Losartan | Tablets | 3/4/2022 |
| Metoclopramide | Inj | 2/28/2022 |
| Metoprolol | Inj | 2/28/2022 |
| Midazolam | Inj | 3/23/2022 |
| Morphine | Inj/PCA vials | 3/14/2022 |
| Nitrofurantoin | Suspension | 3/18/2022 |
| Octreotide | Inj | 3/14/2022 |
| Olanzapine | IM | 2/22/2022 |
| Ondansetron HCL | Inj | 2/22/2022 |
| Pantoprazole | Inj | 3/21/2022 |
| Potassium Cl | Inj | 3/24/2022 |
| Prednisone | Tablets | 1/21/2022 |
| Prochlorperazine | Tablets | 3/14/2022 |
| Temazepam | Capsules | 10/4/2021 |
| Tramadol | Tablets | 3/11/2022 |
| Valsartan | Tablets | 3/3/2022 |
| Vancomycin HCL | Inj | 3/22/2022 |
| Sodium/Dextrose (various) | Inj | 3/22/2022 |
About the Author
David Bougher
Senior VP of Regulatory Affairs
David is a seasoned veteran of the hospice world and an essential member of the Wise Hospice Options clinical team.
Education:
RN, BSN
Experience:
- Former hospice COO
- 20 Years of education & training experience
- 10 Years of experience at Wise Hospice Options

Connect
- (800) 856-9757 Ext: 203
- dbougher@wiseop.com
Follow
Wise Clinical Leadership

Deanna Rice
Vice President
Clinical Services
drice@wiseop.com
(800) 856-9757:
EXT. 216

Tino Vilches
Senior Vice President
Clinical Services
tvilches@wiseop.com
(800) 856-9757:
EXT. 208
Available
Wise Hospice Options Services
Please reach out to your account manager for assistance with maximizing your available services, or for potential upgrades to your current system.
EHR Interface Integrations
Demographic & medication interfaces with over 11 EHR's.
E-Prescribing
Web-based prescribing platform for Schedule II's.
24/7 Support
On-demand clinical or technical assistance fast.
EHR Medication Profiling
Med reviews done inside your EHR system.
Advanced Reporting
Drill down into your pharmacy utilization trends.
Clinical Education
Hospice oreiented courses designed for your hospice staff.
Pharmacy Network Access
Unrestricted access to every pharmacy within our network.
Web-based Refills
Send and identify available refills for your patient population.








